AppliedXL and Bain Bring Wall Street Discipline to the Pharma Industry
Speed defines winners in drug development. Every delay erodes value, burns capital, and prolongs the wait for patients. Yet while biopharma invests heavily in scientific discovery, trial execution remains one of the industry’s biggest blind spots. Most sponsors still rely on manual reviews and fragmented expert networks—tools that are slow, inconsistent, and reactive. It’s like managing a billion-dollar investment portfolio without real-time market data. No investor would tolerate it. Why should drug developers?
To change that, Bain and AppliedXL have built a new framework that fuses real-time AI monitoring with strategic oversight. AppliedXL continuously tracks more than one hundred categories of trial events, from missed milestones to enrollment shifts, and organizes them into a live dashboard of program health. Bain brings the lens to interpret those signals, connect them across programs, and align them with broader market and therapeutic opportunities. Together, this system, called the PRIME framework, detects execution signals early, benchmarks performance against peers, and surfaces insights that help sponsors refine strategies with both clinical relevance and commercial impact. The goal is to transform trial management from reactive firefighting into proactive portfolio management.
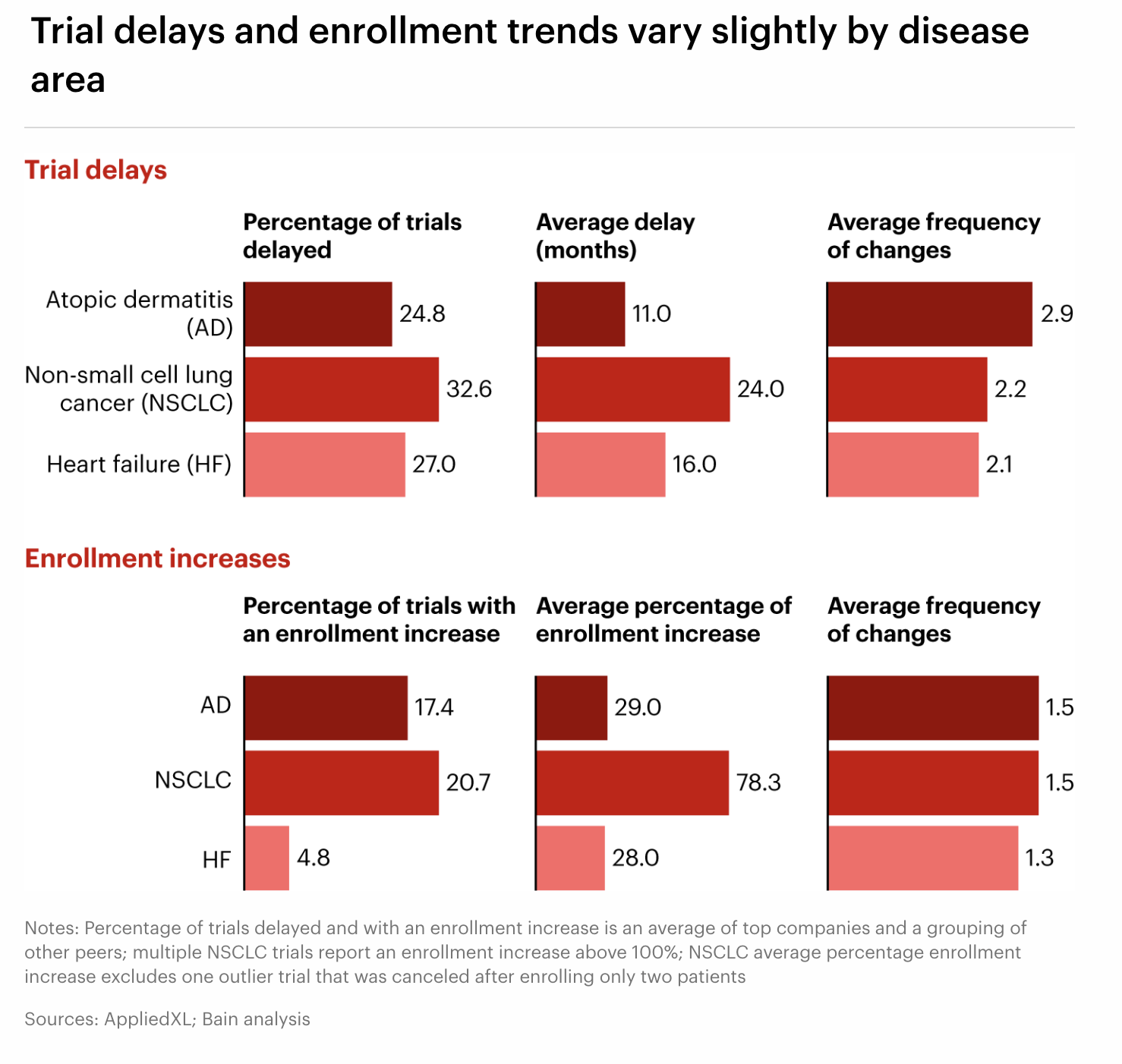
The data shows how execution risk varies across diseases. NSCLC trials are the most vulnerable to long and frequent delays, reflecting the complexity of oncology trial design. Alzheimer’s disease trials, while less delayed, see the highest number of timeline changes, showing how evolving trial designs affect execution. Heart failure trials sit closer to the middle ground, with fewer extremes. These patterns highlight how both scientific complexity and competition for patients drive operational risk.
When viewed at the company level, the differences become even clearer. Some sponsors manage trials consistently across disease areas, while others experience significantly higher execution risk. These variations reflect not only the therapeutic landscape but also organizational posture. Some companies engineer for flawless control, minimizing variance at all costs, while others embrace agility, adapting quickly but accepting more frequent changes.
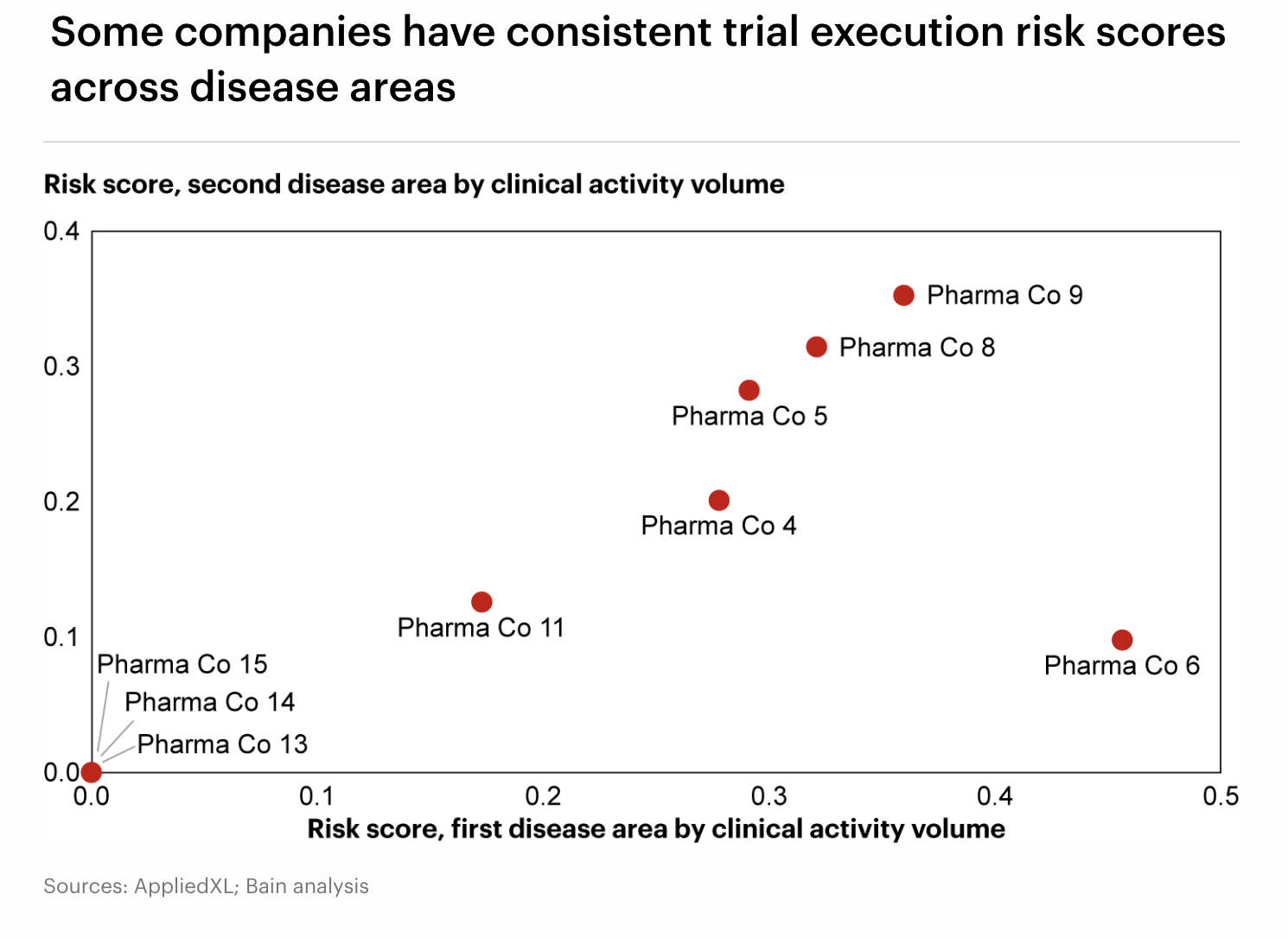
By quantifying these differences, PRIME enables sponsors to see their own posture with fresh clarity, benchmark against peers, and refine strategies to gain an execution edge. For biopharma leaders, the payoff is significant: streamlined operations, reduced development costs, and faster delivery of breakthrough therapies. By combining AppliedXL’s AI-driven intelligence with Bain’s strategic expertise, we are giving the industry a new operating model, one that replaces blind spots with visibility and guesswork with discipline. In a race where precision and speed define success, this framework offers the decisive advantage.